Spinraza (nusinersen) the costly medicine of world treat Musular Dystrophy
Prof Dr,DRAM,HIV /AIDS,HEPATITIS ,SEX DISEASES & WEAKNESS expert,New Delhi,India, +917838059592
The U.S. Food and Drug Administration today approved Spinraza (nusinersen), the first drug approved to treat children and adults with spinal muscular atrophy (SMA), a rare and often fatal genetic disease affecting muscle strength and movement.Spinal muscular atrophy is a genetic disorder that affects the control of muscle movement. It is caused by a loss of specialized nerve cells, called motor neurons, in the spinal cord and the part of the brain that is connected to the spinal cord (the brainstem).
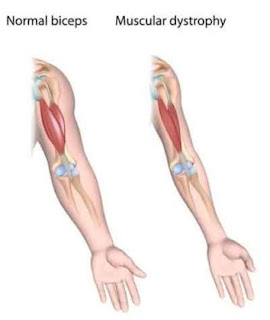 The most common form of SMA (types 1-4) is caused by a defect (mutation) in the SMN1 gene on chromosome 5. (People have two SMN1 genes — one on each chromosome 5.) A mutation in the SMN1 gene leads to a deficiency of a motor neuron protein called SMN, for survival of motor neuron.Spinal muscular atrophy (SMA), also called autosomal recessive proximal spinal muscular atrophy and 5q spinal muscular atrophy in order to distinguish it from other conditions with similar names, is a rare neuromuscular disorder characterised by loss of motor neurons and progressive muscle wasting, often leading to severe crippling muscle wasting involving mid brain too leading to death.
The most common form of SMA (types 1-4) is caused by a defect (mutation) in the SMN1 gene on chromosome 5. (People have two SMN1 genes — one on each chromosome 5.) A mutation in the SMN1 gene leads to a deficiency of a motor neuron protein called SMN, for survival of motor neuron.Spinal muscular atrophy (SMA), also called autosomal recessive proximal spinal muscular atrophy and 5q spinal muscular atrophy in order to distinguish it from other conditions with similar names, is a rare neuromuscular disorder characterised by loss of motor neurons and progressive muscle wasting, often leading to severe crippling muscle wasting involving mid brain too leading to death.
Biogen, which is licensing Spinraza from Ionis Pharmaceuticals, said this week that one dose will have a list price of $125,000. That means the drug will cost $625,000 to $750,000 to cover the five or six doses needed in the first year, and about $375,000 annually after that, to cover the necessary three doses a year.Thus it is the costliest medicine of the World needing hugh money per year to treat such untreatable disease.
Children born with the disease don't make enough of a protein called SMN, which is key to motor development. Spinraza works by amplifying the gene responsible for SMN, thereby boosting its production. The FDA approved the drug to treat both children and adults. Nusinersen is an antisense oligonucleotide (ASO) designed to treat SMA caused by mutations in chromosome 5q that lead to SMN protein deficiency. The drug is used to treat spinal muscular atrophy associated with a mutation in the SMN1 gene. It is administered directly to the central nervous system (CNS) using intrathecal injection.
The lifespan of a Type 2 child varies so widely, there isn't one! They could pass away at an early age or they could live well into adulthood. As with all forms of SMA, weakness increases over time. Type 3 children are diagnosed between 18 months of age and early adolescence.
- Kidney stones universally present hazard in north india,dillution by water prevent it
- Steroid and placebo effect equally for mild persisting asthma with low sputum eosinophils
- Government wants to fix public healthcare staff shortages with ayush docs: will it work?
- Plea in hc for payment of salaries of edmc, north mcd teachers and doctors
- 7 indian pharma companies named in us lawsuit over inflating generic drug prices
- Woman in up dies after explosion in her mouth during treatment,what is diagnosis?
- Woman in up dies after explosion in her mouth during treatment,what is diagnosis?
- Woman in up dies after explosion in her mouth during treatment,what is diagnosis?
- Air pollution ! mothers organising rally in london,anaesthetist choosing gas,will india follow?
- Cardiac arrest is always not sudden as understood -a study

 Comments (
Comments ( Category (
Category ( Views (
Views (