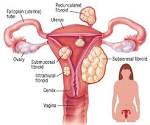
New Uterine Fibroids Oral Treatment Could Replace Surgery
The US Food and Drug Administration (FDA) has accepted the new drug application (NDA) of the investigational drug ulipristal acetate (UA) for the treatment of abnormal bleeding in women with uterine fibroids.While UA has been available in Europe and Canada for several years, the FDA recently notified for this.
“Currently approved treatment options for uterine fibroids are mostly surgical, and these surgeries can take weeks to recover from,” said Ayman Al-Hendy, MD, “That is why ulipristal acetate is a valuable option for treating uterine fibroids. For the first time, doctors and patients will have access to an oral treatment option to manage symptoms. I am optimistic that with a new oral treatment option, fewer women will suffer in silence and leave their uterine fibroids untreated.”
UA belongs to a group of drugs classified as selective progesterone receptor modulators (SPRM), which block progesterone from feeding the fibroids, causing them to shrink. The SPRM acts directly on the progesterone receptors in 3 target tissues - the endometrium, uterine fibroids, and the pituitary gland - to quickly and dramatically decrease and stop vaginal bleeding, which is then typically reduced within 5–6 days.The once-a-day pill is be consumed for 3 months, then stopped for 1 menstrual cycle to allow the endometrium to shed.
The safety and efficacy of UA has been evaluated in VENUS I and VENUS II, 2 phase 3 studies of more than 500 adult women. Additionally, the efficacy of UA has been demonstrated in a series of 4 phase 3 European trials involving more than 1,000 women with uterine fibroids.UA appears to have fewer side effects than the injectable Lupron, the only medication available in the US to shrink fibroids. Side effects such as headaches and hot flashes affected less 10% of women in UA trials.
Lupron effectively sends women into early menopause, which can result in bone loss and hot flashes, as women must often take it with hormone replacement therapy.UA, along with 2 other medications in the pipeline, would provide a new treatment option for a condition often treated with a hysterectomy.Bayer launched a phase 3 clinical trial for vilaprisan, a similar drug, this summer, while AbbVie is currently underway in 2 phase 3 trials for elagolix to treat uterine fibroids and endometriosis.
Similar to Lupron, elagolix decreases sex hormones that causes fibroids to grow, however it is fast acting and easier to reverse the effects if stopped. Studies show it significantly reduces heavy menstrual bleeding and decreases the thickness of the endometrium by 6 months.While the most common treatment option for uterine fibroids is a hysterectomy, women who wish to still undergo childbirth can have a myomectomy, a surgery that removes fibroids and keeps the uterus intact.
- Kidney stones universally present hazard in north india,dillution by water prevent it
- Steroid and placebo effect equally for mild persisting asthma with low sputum eosinophils
- Government wants to fix public healthcare staff shortages with ayush docs: will it work?
- Plea in hc for payment of salaries of edmc, north mcd teachers and doctors
- 7 indian pharma companies named in us lawsuit over inflating generic drug prices
- Woman in up dies after explosion in her mouth during treatment,what is diagnosis?
- Woman in up dies after explosion in her mouth during treatment,what is diagnosis?
- Woman in up dies after explosion in her mouth during treatment,what is diagnosis?
- Air pollution ! mothers organising rally in london,anaesthetist choosing gas,will india follow?
- Cardiac arrest is always not sudden as understood -a study

 Comments (
Comments ( Category (
Category ( Views (
Views (